Learning Outcomes
i. Define and explain the concept of electrochemical processes, providing a foundation for understanding the conversion of chemical energy to electrical energy.
ii. Identify the key components of electrochemical cells, including electrodes, electrolytes, and the salt bridge.
iii. Explain the mechanism of electron flow in electrochemical cells, including the roles of oxidation and reduction.
iv. Differentiate between galvanic cells and electrolytic cells based on their energy conversion processes.
v. Apply the concept of electrochemical cells to real-world applications, such as batteries, fuel cells, and electrolysis.
Introduction
In the intricate realm of chemistry, where energy transformation reigns supreme, electrochemical processes emerge as a captivating spectacle of chemical energy conversion into electrical energy. This lesson will delve into the nature of electrochemical processes, empowering you to comprehend the intricate dance of electrons that powers our world.
i. Electrochemical Cells: The Powerhouses of Energy Transformation
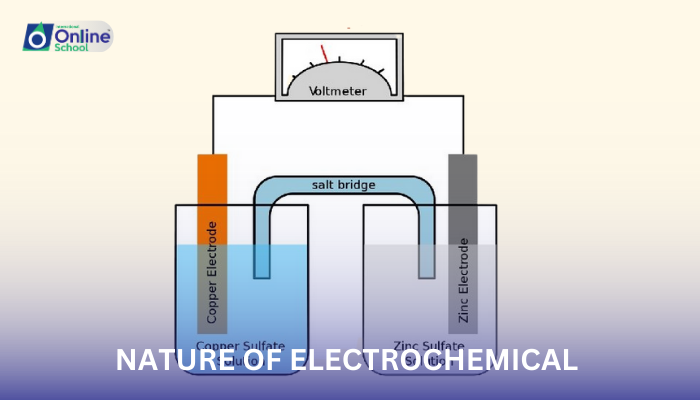
Electrochemical cells, the heart of electrochemical processes, are ingenious devices that harness the power of chemical reactions to generate electrical energy. These fascinating contraptions consist of essential components:
Electrodes: The two conductive surfaces where redox reactions occur. The anode is the site of oxidation, where electrons are released, while the cathode is the site of reduction, where electrons are accepted.
Electrolyte: A conductive solution containing ions that allow the movement of electrons between the electrodes.
Salt bridge: A porous barrier that allows ion flow between the electrolyte solutions in the anode and cathode compartments, maintaining electrical neutrality.
ii. The Mechanism of Electron Flow: A Dance of Oxidation and Reduction
At the heart of electrochemical processes lies the silent dance of electrons. In a galvanic cell, a spontaneous chemical reaction drives the flow of electrons from the anode to the cathode, generating electrical energy. This electron flow is orchestrated by the redox reactions occurring at the electrodes:
Anode: Oxidation occurs, where a species loses electrons, increasing its oxidation state.
Cathode: Reduction occurs, where a species gains electrons, decreasing its oxidation state.
iii. Galvanic Cells vs. Electrolytic Cells: A Matter of Energy Conversion
Electrochemical cells can be classified into two main types: galvanic cells and electrolytic cells. These cells differ in the direction of electron flow and the energy source:
Galvanic cells: Spontaneous chemical reactions drive the flow of electrons, generating electrical energy.
Electrolytic cells: An external electrical energy source is applied to force the flow of electrons in a non-spontaneous direction, causing chemical reactions to occur.
iv. Real-World Applications: A Symphony of Electrochemical Processes
Electrochemical processes have revolutionized our world, powering our devices, providing clean energy solutions, and shaping various industries. From the batteries that fuel our smartphones to the fuel cells that generate electricity without emissions, electrochemical processes play a pivotal role in modern society.
Electrochemical processes, the captivating interplay of chemical energy and electrical energy, have transformed our world, providing a glimpse into the intricate dance of electrons that powers our devices and drives innovation. Understanding the nature of electrochemical cells, the mechanism of electron flow, and the distinction between galvanic and electrolytic cells empowers us to appreciate the significance of these processes in shaping our world.